
They fuse to become helium that is lower on the curve. The left side of Figure 4 shows that isotopes of hydrogen are located at the highest and steepest part the energy-valley curve. Whereas fission is the breaking apart of atomic nuclei, nuclear fusion is the combining (fusing) of atomic nuclei. A possible long-range source of energy now being vigorously studied makes use of the left side of the energy valley-nuclear fusion-the opposite of nuclear fission. In today’s world, all nuclear power plants employ fission. That lowered mass per nucleon converts to energy via E = mc 2. The graph in Figure 4 tells us that the fission of uranium produces elements lower on the curve. We can think of this curve as an “energy valley” that starts at the highest point (hydrogen), slopes steeply down to level off at the lowest point (iron), and then slopes gradually upward to uranium. It is essential to understanding the energy associated with all nuclear processes-particularly nuclear fission and fusion. I regard Figure 4 as the most important graph in a study of nuclear physics. Conversely, when nucleons bind together to form a nucleus, they release energy, called binding energy, losing the equivalent mass.

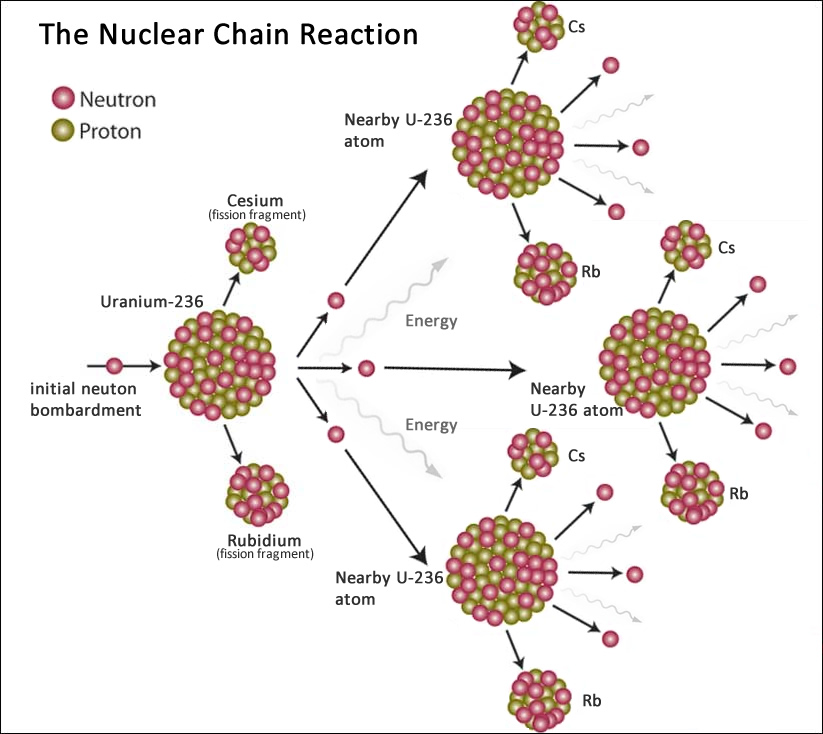
#FISSION REACTION FREE#
So we see that a free nucleon has more mass than a nucleon locked inside the nucleus. What becomes of this work? In accord with E = mc 2, the energy that is expended adds to the mass of the liberated nucleon. On the atomic scale, appreciable force is required to pull a nucleon out of an atomic nucleus through a sufficient distance to overcome the attractive strong force that holds the nucleus together. Recall that work is expended energy equal to force × distance. This is called the mass defect.Ĭonsider the work required to separate nucleons from a nucleus (Figure 2). Two concepts are central to both nuclear fission and fusion: First, the mass of a nucleus is less than the sum of the masses the nucleons would have if they were free. Nuclear mass versus the mass of free nucleons E = mc 2 is the key to understanding why and how energy is released in nuclear reactions. The quantity c 2 is the proportionality constant between E and m, a huge number that reveals how a tiny amount of mass converts to enormous energy. In this equation, E stands for the energy that any mass has at rest, m stands for mass, and c is the speed of light. Mass and energy are two sides of the same coin, stated in Einstein’s celebrated equation, E = mc 2. Where does this nuclear energy come from? The answer has to do with a discovery made by Albert Einstein in 1905.Įinstein discovered an amazing connection between mass and energy: Mass is actually “congealed” energy. In comparison, about 2 eV of energy per molecule is involved in chemical reactions. But most astounding is the energy released, some 200 million electron volts (eV) of energy per nucleus (an electron volt is a unit of energy equal to 1.6 × 10 -19 joule).


 0 kommentar(er)
0 kommentar(er)
